Introduction of Cations and Anions, Difference between cations and anions, Cations and anions notes, Cations and anions formula, cations examples, 10 examples of cations and anions, Cations and anions class 11, Cations and anions list, Cations and anions table
Cations and anions table
When it comes to the fascinating world of chemistry, one of the fundamental concepts you’ll encounter is the distinction between cations and anions. These two types of ions play a crucial role in the formation of ionic compounds and are essential for understanding the behavior of various chemical substances. In this blog post, we’ll delve deep into the definitions, properties, and differences between cations and anions, shedding light on their vital roles in chemistry.
What is a Cation?
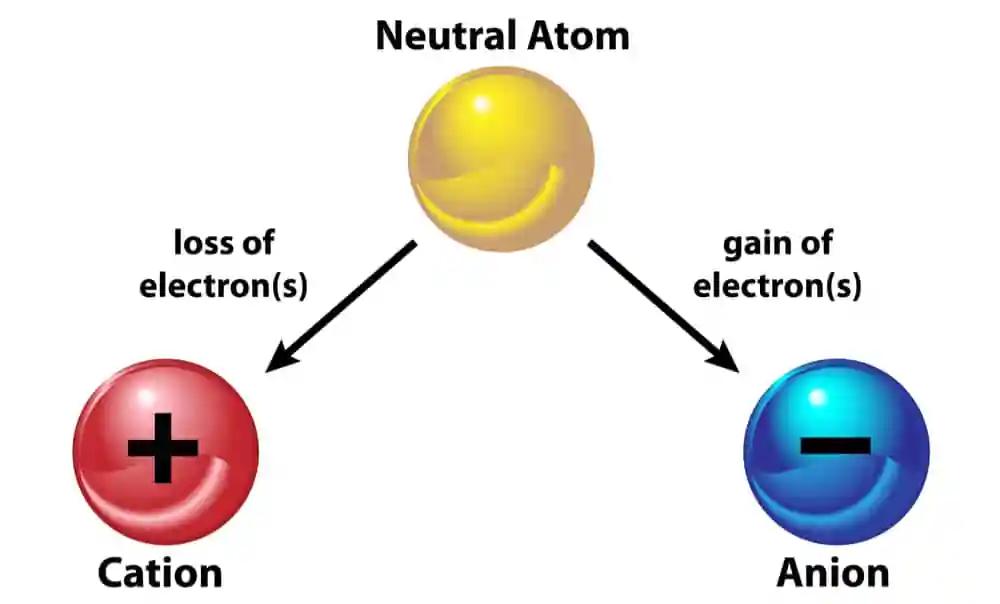
Let’s begin by unraveling the mystery of cations. A cations is a positively charged ion that forms when an atom loses one or more electrons. This loss of electrons results in an excess of protons, giving the ion a net positive charge. Cations are often formed by metals and are characterized by their tendency to donate electrons to other atoms during chemical reactions.
For example, consider the sodium (Na) atom. Sodium has 11 electrons and is located in Group 1 of the periodic table. When it reacts with another element or compound, it readily loses its outermost electron to achieve a stable electron configuration. As a result, sodium forms a sodium cation, denoted as Na+, with a +1 charge. This cation is now attracted to negatively charged ions, or anions, due to their electrostatic attraction.
What is an Anion?
On the flip side, anions are negatively charged ions that form when an atom gains one or more electrons. This gain of electrons results in an excess of electrons, giving the ion a net negative charge. Anions are typically formed by nonmetals, and they exhibit a strong tendency to attract positively charged ions, or cations.
Let’s take the example of chlorine (Cl), a nonmetal in Group 17 of the periodic table. Chlorine has 17 electrons, and when it reacts with another element or compound, it readily gains one electron to achieve a stable electron configuration. As a result, chlorine forms a chloride anion, denoted as Cl-, with a -1 charge. This anion now exhibits a strong electrostatic attraction to positively charged cations, forming ionic compounds when they combine.
Cation vs. Anion: Key Differences
Now that we have a clear understanding of what cations and anions are, let’s highlight some key differences between the two:
- Charge: Cations have a positive charge, while anions have a negative charge.
- Formation: Cations are formed when atoms lose electrons, while anions are formed when atoms gain electrons.
- Metals vs. Nonmetals: Cations are typically formed by metals, whereas anions are predominantly formed by nonmetals.
- Electrostatic Attraction: Cations are attracted to anions due to their opposite charges, resulting in the formation of ionic compounds.
- Chemical Reactivity: Cations tend to be more reactive by donating electrons, while anions are reactive by accepting electrons.
| Characteristic | Cations | Anions |
| Charge | Positively charged (+) | Negatively charged (-) |
| Formation | Lose one or more electrons | Gain one or more electrons |
| Typical Elements | Metals | Nonmetals |
| Electrostatic Attraction | Attracted to anions | Attracted to cations |
| Chemical Reactivity | Tend to donate electrons | Tend to accept electrons |
| Example | Sodium ion (Na+) | Chloride ion (Cl-) |
| Role in Ionic Compounds | Combine with anions to form | Combine with cations to form |
| Everyday Importance | Essential for biological | Essential for various |
Ionic Compounds: The Marriage of Cations and Anions
The interaction between cations and anions is the foundation of ionic compounds. These compounds are composed of a combination of positively charged cations and negatively charged anions held together by strong electrostatic attractions. The ratio of cations to anions in an ionic compound is always such that the overall charge is neutral.
For instance, sodium chloride (NaCl) is a well-known ionic compound formed by the combination of sodium cations (Na+) and chloride anions (Cl-). The electrostatic attraction between these ions results in the formation of a crystalline structure with distinct properties, such as high melting and boiling points and electrical conductivity when dissolved in water.
The Importance of Cations and Anions in Everyday Life
Cations and anions play a vital role not only in the field of chemistry but also in our daily lives. They are essential for various biological processes, including nerve impulses and muscle contractions in the human body. Additionally, they are crucial in environmental chemistry, as they influence the behavior of ions in soil and water, affecting plant growth and water quality.
Furthermore, many household items, such as batteries and cleaning products, rely on the movement of cations and anions to function effectively. Understanding the behavior of these ions allows us to develop innovative technologies and solutions that enhance our quality of life.
Conclusion
In conclusion, cations and anions are fundamental concepts in chemistry that are essential for understanding the behavior of chemical substances. Cations, with their positive charge, are formed by the loss of electrons, primarily by metals. Anions, on the other hand, are negatively charged ions formed by the gain of electrons, mainly by nonmetals. The interaction between these oppositely charged ions leads to the formation of ionic compounds, which have widespread applications in various fields.
So, the next time you encounter salt on your dining table or use a battery-powered device, remember that cations and anions are working behind the scenes, contributing to the chemistry that shapes our world. These charged ions are indeed the unsung heroes of the molecular world, and understanding their roles is a step toward appreciating the beauty of chemistry in our everyday lives.